In patients with schizophrenia or schizoaffective disorder, Versacloz offers the...
Proven efficacy of clozapine in reducing the risk of recurrent suicidal behavior1
Versacloz is indicated for reducing the risk of recurrent suicidal behavior in at-risk patients with schizophrenia or schizoaffective disorder1
Study design1
- The effectiveness of clozapine in reducing the risk of recurrent suicidal behavior was assessed in the International Suicide Prevention Trial (InterSePTTM)
- InterSePTTM was a prospective, randomized, international, parallel-group comparison of clozapine versus olanzapine in patients with schizophrenia or schizoaffective disorder. All patients were seen weekly for 6 months and bi-weekly for 18 months. Clinicians could make interventions necessary to prevent the occurrence of suicide attempts. Suicidal behavior was assessed at each visit2
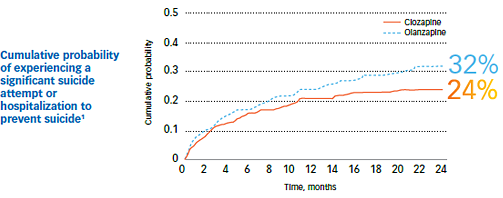
Study outcomes1
- The cumulative probability of experiencing a significant suicide attempt or hospitalization due to suicide risk was lower for clozapine patients (24%) than for olanzapine patients (32%)1
- Patients treated with clozapine had a statistically significant longer delay in the time to recurrent suicidal behavior in comparison with olanzapine1
- This result should be interpreted only as evidence of the effectiveness of clozapine in delaying time to recurrent suicidal behavior and not a demonstration of the superior efficacy of clozapine over olanzapine
Important Safety Information
Eosinophilia
Eosinophilia, defined as a blood eosinophil count of greater than 700/µL, has occurred with clozapine treatment. In clinical trials, approximately 1% of patients developed eosinophilia. Clozapine-related eosinophilia usually occurs during the first month of treatment. In some patients, it has been associated with myocarditis, pancreatitis, hepatitis, colitis, and nephritis. Such organ involvement could be consistent with a drug reaction with eosinophilia and systemic symptoms syndrome (DRESS), also known as drug induced hypersensitivity syndrome (DIHS). If eosinophilia develops during VERSACLOZ treatment, evaluate promptly for signs and symptoms of systemic reactions, such as rash or other allergic symptoms, myocarditis, or other organ-specific disease associated with eosinophilia. If clozapine-related systemic disease is suspected, discontinue VERSACLOZ immediately.
If a cause of eosinophilia unrelated to clozapine is identified (e.g., asthma, allergies, collagen vascular disease, parasitic infections, and specific neoplasms), treat the underlying cause and continue VERSACLOZ.
Clozapine-related eosinophilia has also occurred in the absence of organ involvement and can resolve without intervention. There are reports of successful rechallenge after discontinuation of clozapine, without recurrence of eosinophilia. In the absence of organ involvement, continue VERSACLOZ under careful monitoring. If the total eosinophil count continues to increase over several weeks in the absence of systemic disease, the decision to interrupt VERSACLOZ therapy and rechallenge after the eosinophil count decreases should be based on the overall clinical assessment, in consultation with an internist or hematologist.
QT Interval Prolongation
QT prolongation, Torsades de Pointes and other life-threatening ventricular arrhythmias, cardiac arrest, and sudden death have occurred with clozapine treatment. When prescribing VERSACLOZ, consider the presence of additional risk factors for QT prolongation and serious cardiovascular reactions. Conditions that increase these risks include the following: history of QT prolongation, long QT syndrome, family history of long QT syndrome or sudden cardiac death, significant cardiac arrhythmia, recent myocardial infarction, uncompensated heart failure, treatment with other medications that cause QT prolongation, treatment with medications that inhibit the metabolism of VERSACLOZ, and electrolyte abnormalities.
Prior to initiating treatment with VERSACLOZ, perform a careful physical examination, medical history, and concomitant medication history. Consider obtaining a baseline ECG and serum chemistry panel. Correct electrolyte abnormalities. Discontinue VERSACLOZ if the QTc interval exceeds 500 msec. If patients experience symptoms consistent with Torsades de Pointes or other arrhythmias, (e.g., syncope, presyncope, dizziness, or palpitations), obtain a cardiac evaluation and discontinue VERSACLOZ.
Use caution when administering concomitant medications that prolong the QT interval or inhibit the metabolism of VERSACLOZ. Drugs that cause QT prolongation include: specific antipsychotics (e.g., ziprasidone, iloperidone, chlorpromazine, thioridazine, mesoridazine, droperidol, pimozide), specific antibiotics (e.g., erythromycin, gatifloxacin, moxifloxacin, sparfloxacin), Class 1A antiarrhythmic medications (e.g., quinidine, procainamide) or Class III antiarrhythmic (e.g., amiodarone, sotalol), and others (e.g., pentamidine, levomethadyl acetate, methadone, halofantrine, mefloquine, dolasetron mesylate, probucol or tacrolimus). VERSACLOZ is primarily metabolized by CYP isoenzymes 1A2, 2D6, and 3A4. Concomitant treatment with inhibitors of these enzymes can increase the concentration of VERSACLOZ [see Drug Interactions (7.1) and Clinical Pharmacology (12.3) of the Prescribing Information].
Hypokalemia and hypomagnesemia increase the risk of QT prolongation. Hypokalemia can result from diuretic therapy, diarrhea, and other causes. Use caution when treating patients at risk for significant electrolyte disturbance, particularly hypokalemia. Obtain baseline measurements of serum potassium and magnesium levels, and periodically monitor electrolytes. Correct electrolyte abnormalities before initiating treatment with VERSACLOZ.
Click here for additional safety information.
References: 1. VERSACLOZ Full Prescribing Information, Douglas Pharmaceuticals America Ltd. 2025 2. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia. International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60:82-91.
United States.