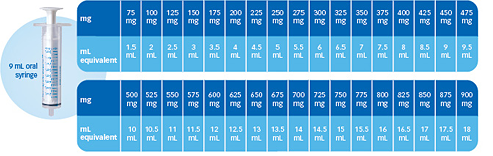
Measuring Versacloz to the clozapine dose prescribed
Versacloz is measured and administered using an
oral syringe—no need to mix or match clozapine tablets
- Provides a highly concentrated, low-volume suspension:
1 mL of Versacloz = 50 mg of clozapine1 - Requires no refrigeration
- Easy conversion from mg to mL
Before you administer Versacloz, use this chart to convert
mg to mL
This is being provided as a general reference tool. For more information on dosing of Versacloz, click here.
Use of oral syringes
- When administering a dose of 50 mg (1 mL) or less, use the smaller 1 mL oral syringe
- If you are administering a dose of more than 50 mg (1 mL), it is important that you use the larger 9 mL oral syringe
Important Safety Information
Recurrence of Psychosis and Cholinergic Rebound after Abrupt Discontinuation of VERSACLOZ
If abrupt discontinuation of VERSACLOZ is necessary (because of severe neutropenia or another medical condition, for example) [see Dosage and Administration (2.6), Warnings and Precautions (5.1) of the Prescribing Information], monitor carefully for the recurrence of psychotic symptoms and adverse reactions related to cholinergic rebound, such as profuse sweating, headache, nausea, vomiting, and diarrhea.
ADVERSE REACTIONS
Most common adverse reactions (≥5%) were: CNS reactions (sedation, dizziness/vertigo, headache, and tremor); cardiovascular reactions (tachycardia, hypotension, and syncope); autonomic nervous system reactions (hypersalivation, sweating, dry mouth, and visual disturbances); gastrointestinal reactions (constipation and nausea); and fever.
Click here for additional safety information.
Reference: 1. Versacloz® Prescribing Information, Douglas Pharmaceuticals America Ltd. 2025
United States.