For your patients with treatment-resistant schizophrenia…
Change course with the proven efficacy of clozapine
VERSACLOZ offers the proven efficacy of clozapine,1
a recognized option2 in treatment-resistant schizophrenia
Study design1
- The efficacy of clozapine, the active molecule in VERSACLOZ oral suspension, was established in a 6-week, randomized, double-blind, active-controlled study comparing clozapine and chlorpromazine in patients with a DSM-III diagnosis of schizophrenia who had inadequate response to at least 3 different antipsychotics (from at least 2 different chemical classes) during the preceding 5 years
- The primary endpoint was treatment response, predefined as a decrease in Brief Psychiatric Rating Scale (BPRS) score of at least 20% and either (1) a Clinical Global Impressions – Severity Scale (CGI-S) score of ≤3 (mildly ill), or (2) a BPRS score of ≤35, at the end of 6 weeks of treatment
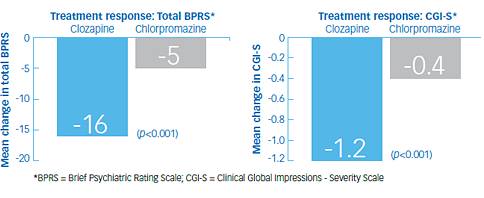
Study outcomes1
- At the end of 6 weeks, 30% of the clozapine group responded to treatment, and 4% of the chlorpromazine group responded to treatment (p<0.001). The mean change in total BPRS score was -16 and -5 in the clozapine and chlorpromazine groups, respectively
- Secondary analyses showed a mean reduction of -5 in the clozapine group and -2 in the chlorpromazine group on the cluster of 4 key BPRS item scores of conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content; and a mean change in CGI-S of -1.2 in in the clozapine group and -0.4 in the chlorpromazine group (p<0.001 in each analysis)
Important Safety Information
Contraindications include:
- Known hypersensitivity to clozapine (e.g., photosensitivity, vasculitis, erythema multiforme, or Stevens-Johnson Syndrome) or any other component of VERSACLOZ
Severe Neutropenia
VERSACLOZ has caused severe neutropenia (absolute neutrophil count (ANC) less than 500/μL) [see Adverse Reactions (6.1, 6.2) of the Full Prescribing Information] and is associated with an increased risk of serious and potentially fatal infections. Severe neutropenia occurred in a small percentage of VERSACLOZ-treated patients. The risk of severe neutropenia appears greatest during the first 18 weeks of VERSACLOZ treatment. The mechanism by which VERSACLOZ causes neutropenia is unknown. Neutropenia is not dose dependent.
Consider a hematology consultation before initiating VERSACLOZ treatment or during treatment.
ANC Monitoring and Dosage Modifications
Prior to initiating VERSACLOZ treatment, obtain a baseline ANC. VERSACLOZ initiation is not recommended in patients with a baseline ANC less than 1500/μL. Throughout VERSACLOZ treatment, regularly monitor ANC.
Table 1 under the Dosage and Administration section of this website provides recommendations for dosage modifications (dosage interruption and treatment discontinuation), based on ANC levels, during VERSACLOZ treatment and frequency of ANC monitoring [see Dosage and Administration (2.4) in the Full Prescribing Information].
ANC Monitoring and Dosage Modification in Patients with Benign Ethnic Neutropenia
Patients with Benign Ethnic Neutropenia (BEN) (also known as Duffy-null associated neutrophil count) generally have lower baseline neutrophil counts but they are not at higher risk for developing infections, and they are not at increased risk for developing VERSACLOZ-induced neutropenia.
For patients with documented BEN, obtain at least two baseline ANC levels prior to VERSACLOZ initiation. VERSACLOZ initiation is not recommended in patients with BEN with an ANC less than 1000/μL. There are different ANC dosage modification recommendations in VERSACLOZ-treated patients with BEN due to their lower baseline ANC levels.
Table 2 under the Dosage and Administration section of this website provides recommendations on dosage modifications (dosage interruption and treatment discontinuation), based on ANC monitoring, during VERSACLOZ treatment in patients with BEN and recommended frequency of ANC testing [see Dosage and Administration (2.5) in the Full Prescribing Information].
Management of VERSACLOZ-Treated Patients Who Develop a Fever
For patients who develop a fever during VERSACLOZ treatment:
- Interrupt VERSACLOZ in those who develop a temperature of 101.3 °F (38.5 °C) or greater and obtain an ANC level.
- If the ANC is less than 1000/μL in patients without BEN, initiate appropriate workup and treatment for infection. Table 1 under the Dosage and Administration section of this website provides dosage modifications based on ANC monitoring [see Dosage and Administration (2.4) of the Full Prescribing Information].
In patients with fever and a normal neutrophil count, see Warnings and Precautions (5.11) for neuroleptic malignant syndrome and Warnings and Precautions (5.13) for fever, of the Full Prescribing Information.
Restarting VERSACLOZ in Patients Who Recovered from Severe Neutropenia
Generally, do not rechallenge patients with VERSACLOZ in those who experienced severe neutropenia. However, for some patients who had resolution of their VERSACLOZ-related severe neutropenia after stopping VERSACLOZ, the risk of schizophrenia exacerbation from not restarting VERSACLOZ treatment may be greater than the risk of neutropenia reoccurrence from restarting VERSACLOZ (e.g., patients who have no treatment options other than VERSACLOZ).
Concomitant Use of VERSACLOZ with Other Drugs Known to Cause Neutropenia
If VERSACLOZ is used concomitantly with another drug known to cause neutropenia, consider more frequently ANC monitoring than the recommendations provided in Table 1 and Table 2 (refer to the Dosage and Administration section of this website).
Click here for additional safety information.
References: 1. VERSACLOZ Full Prescribing Information, Douglas Pharmaceuticals America Ltd. 2025 2. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Schizophrenia, Third Edition. American Psychiatric Association. 2021.
United States.